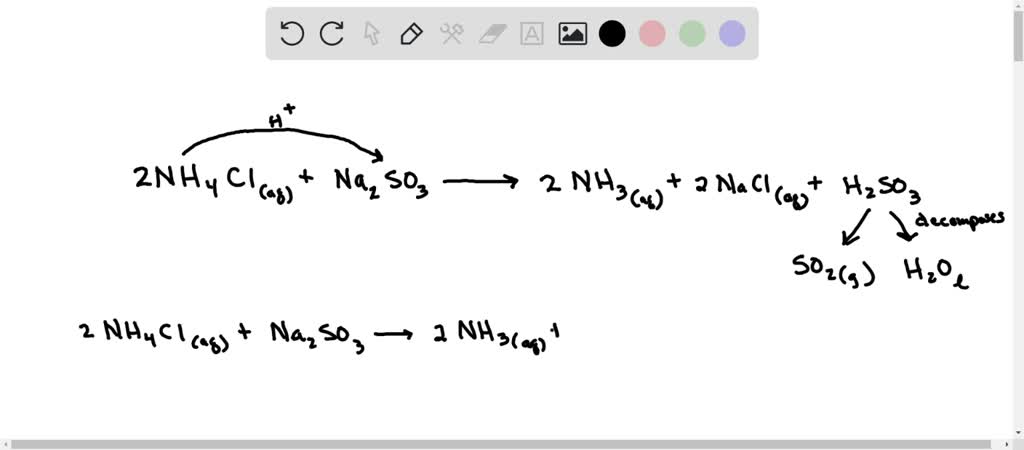
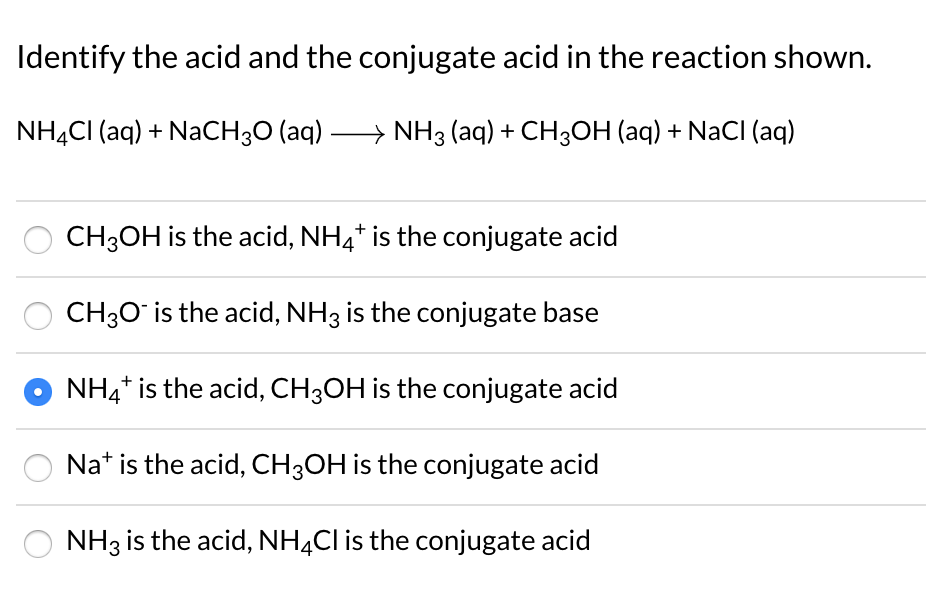
How to predict whether an aqueous solution of the following salts will be acidic, basic, or neutral? Justify your answers: KCl, NaCN, NH4NO3, and NH4C2H3O2 NH4CN - Quora

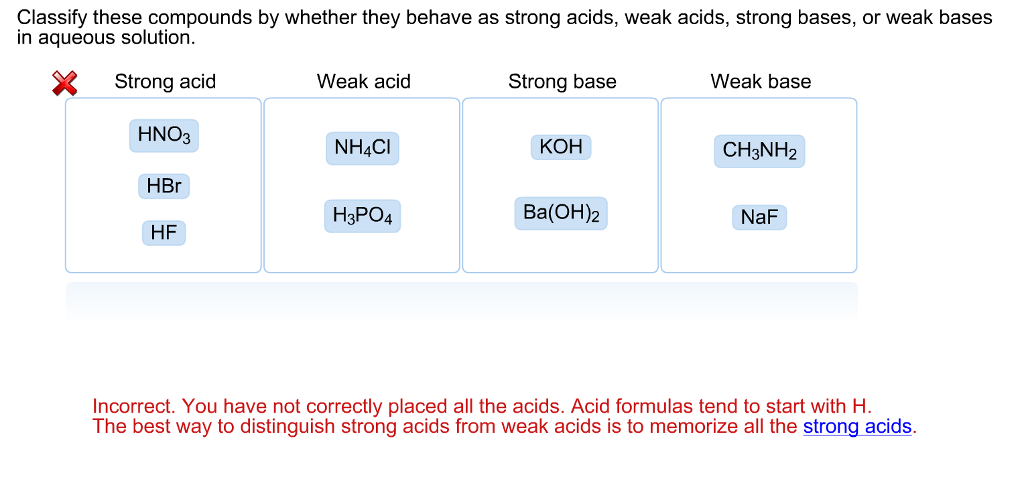
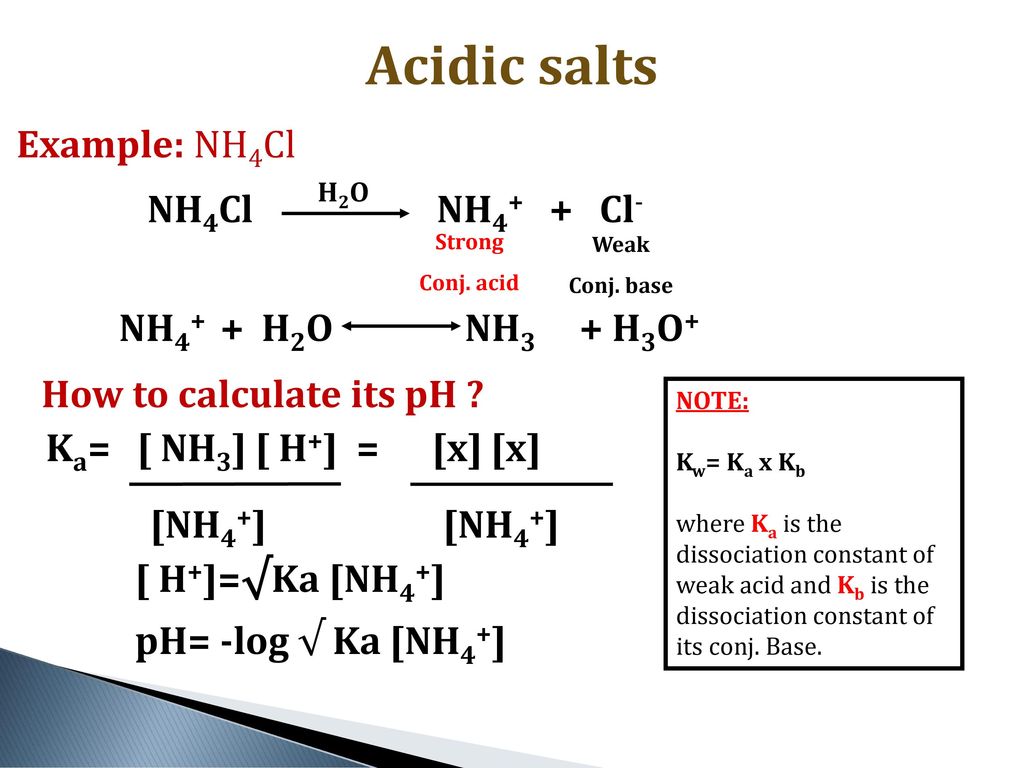
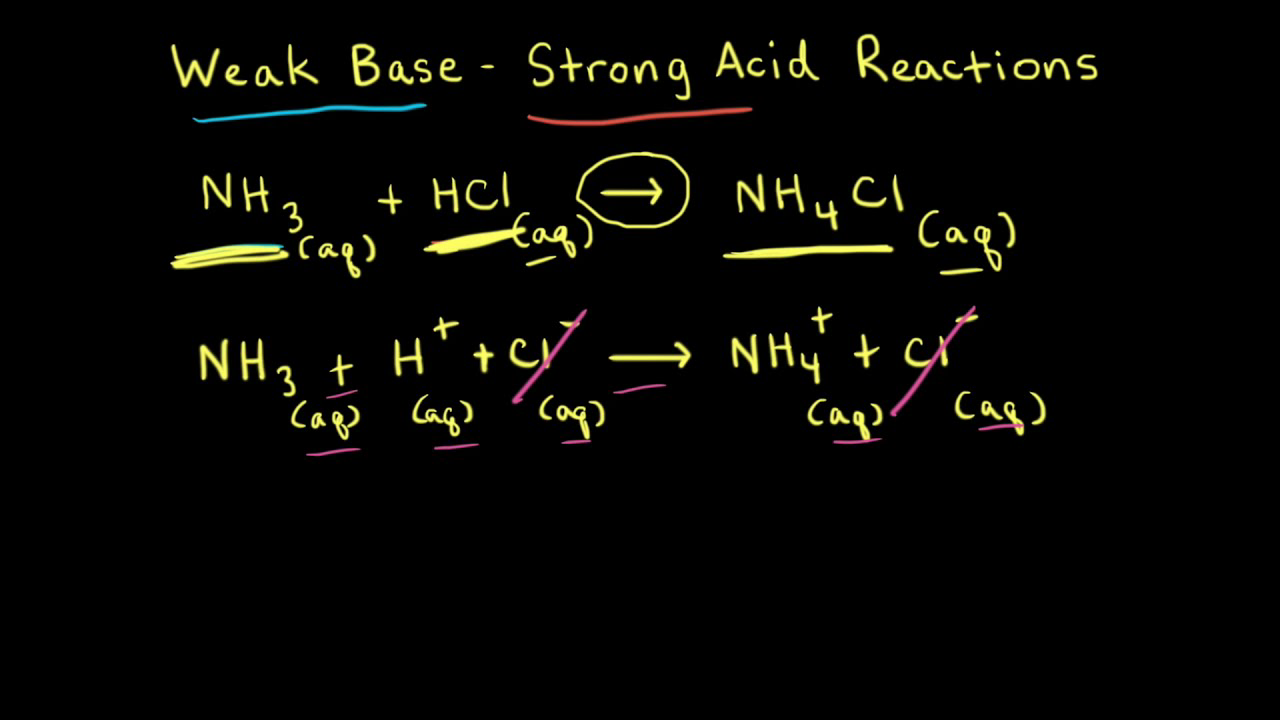
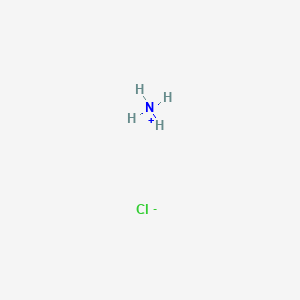
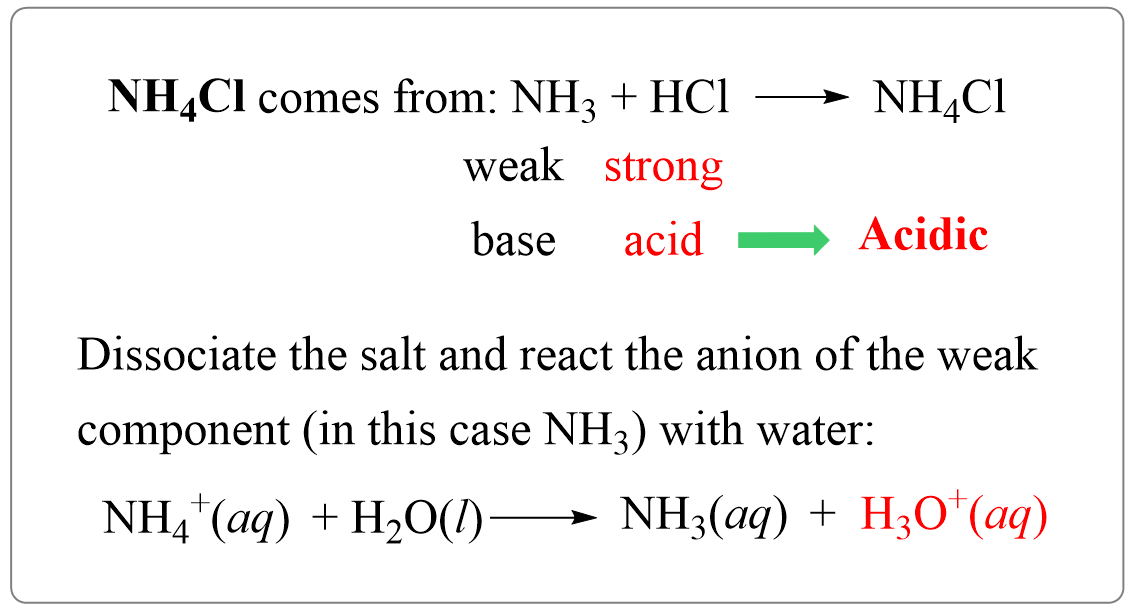
NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in

NH3 is a weak base (Kb = 1.8 x 10-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.014 M in

NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in
CHEM 1332 (A.M. Guloy) CHEMICAL EQUILIBRIA--ACID/BASE Acid/base problems may fall into 4 categories: strong acid/base, weak acid

Why an aqueous solution of NH4Cl is acidic while that of HCOOK is basic - Chemistry - Ionic Equilibria - 16488273 | Meritnation.com

pH calculation of a buffer solution made from a weak base and its conjugate acid (salt form) - YouTube