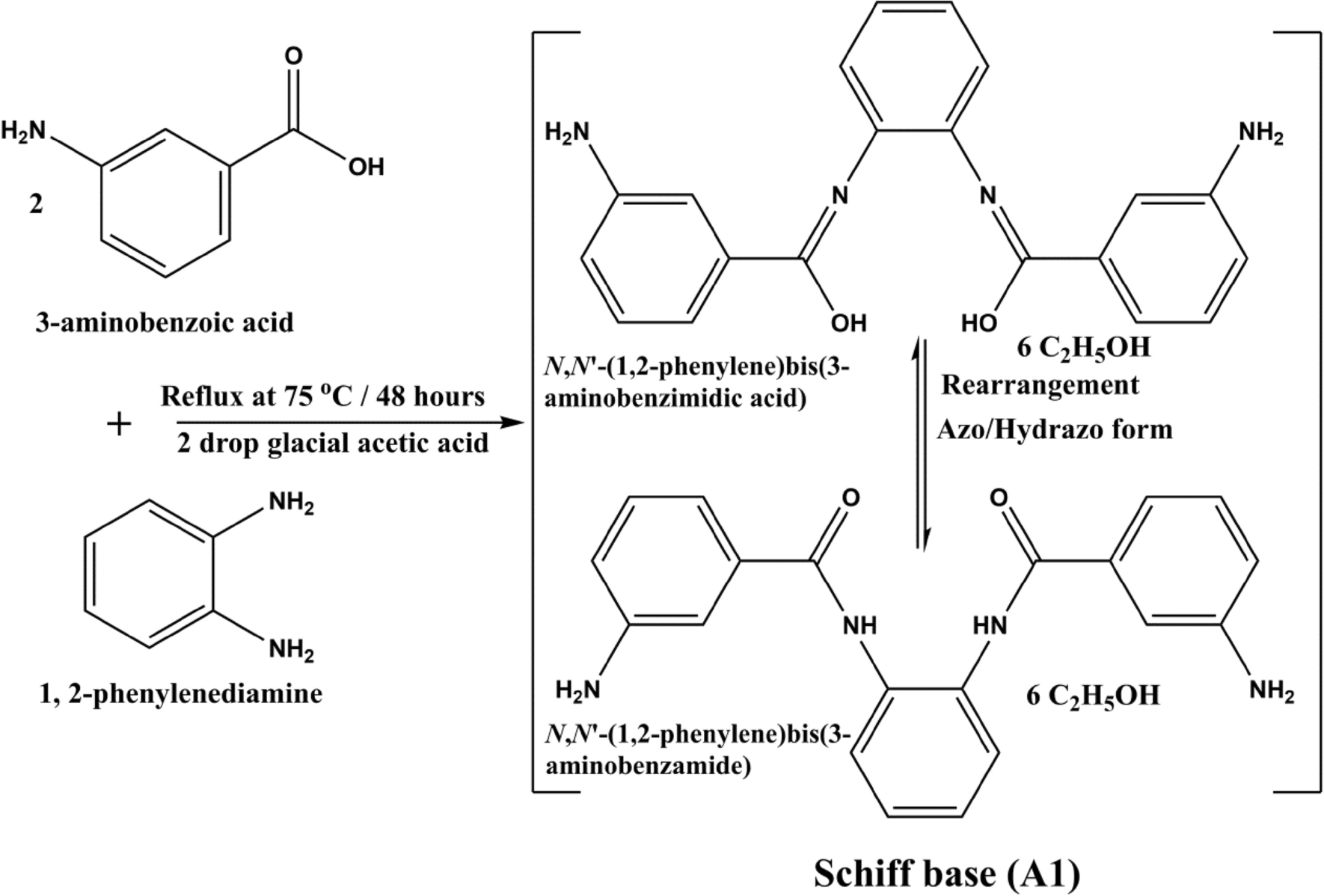
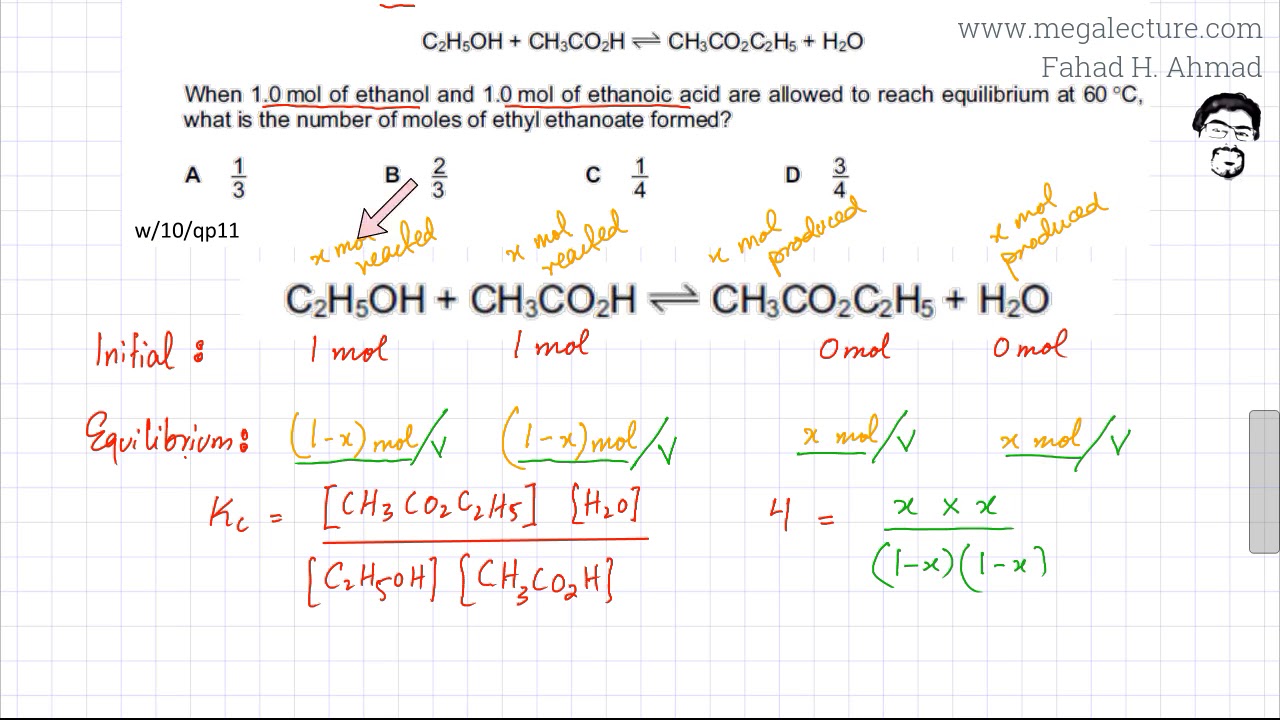
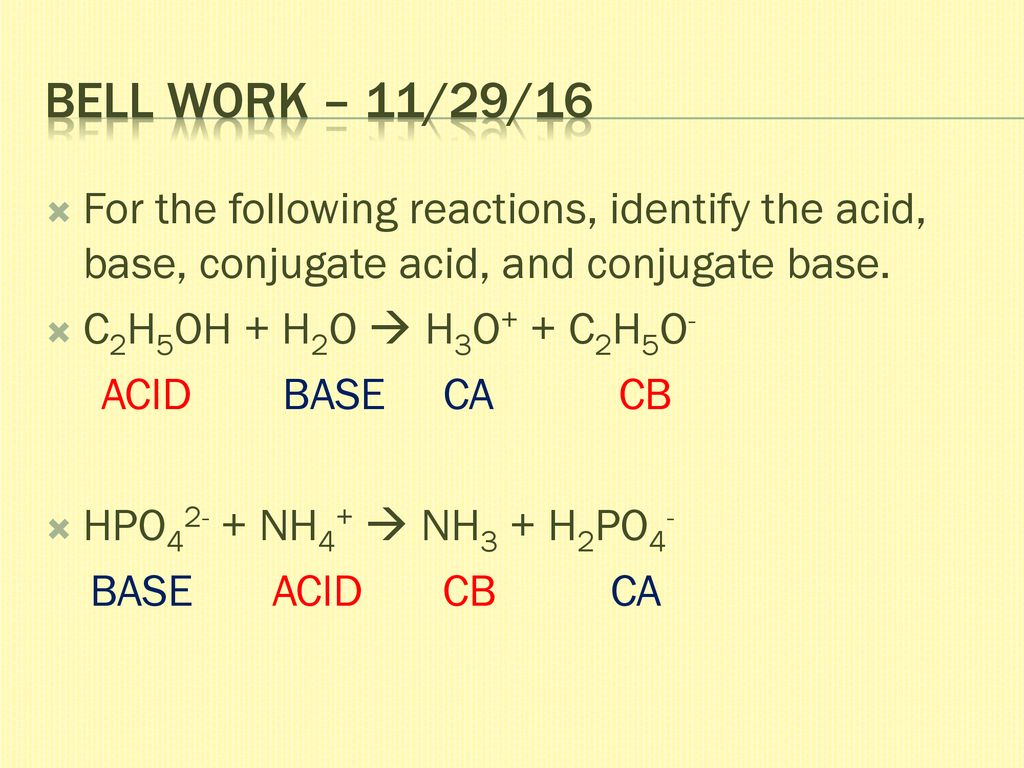
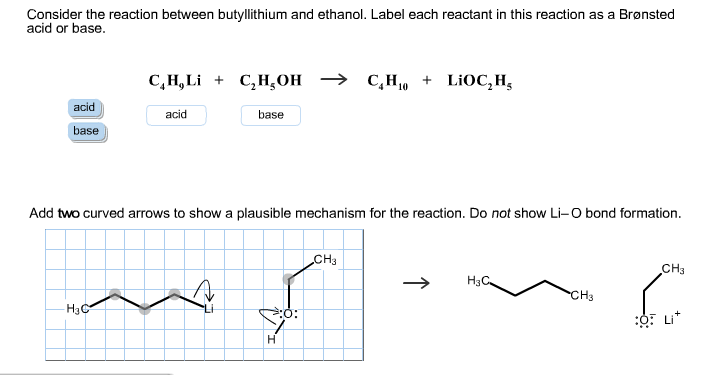
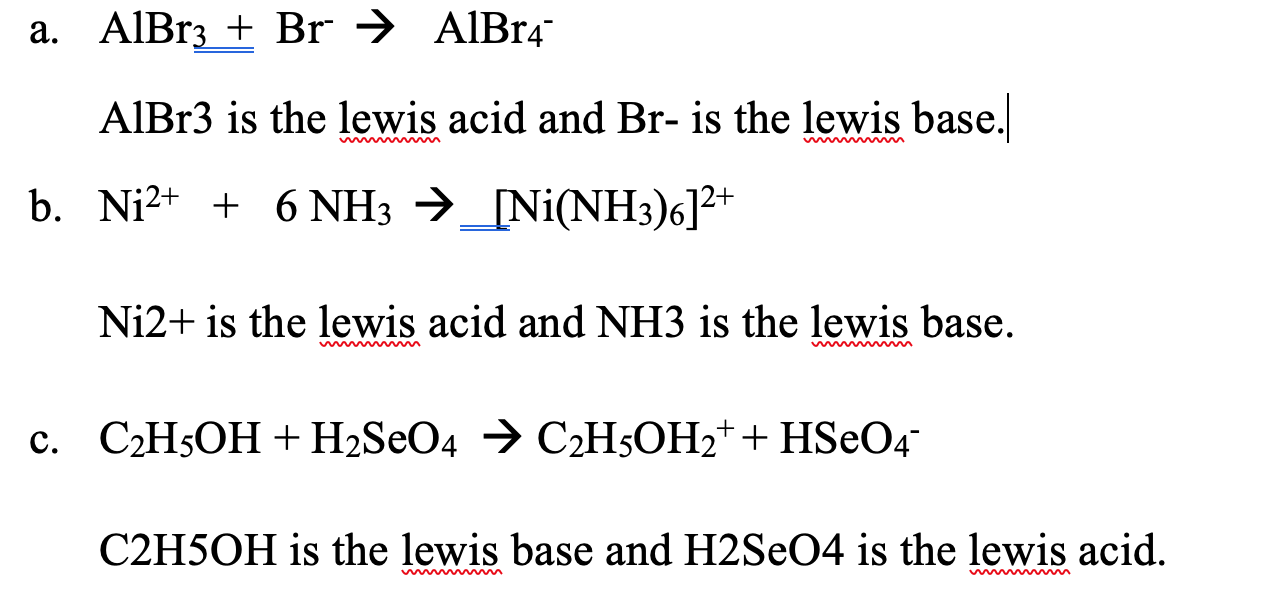SOLVED: C2H5OH is an substance and a a. organi, non, electrolyte b. organic, strong electrolyte c. strong base, strong electrolyte d. strong acid, strong electrolyte

Reagents and conditions: (a) CCl3CH(OH)2, NH2OH. HCl, 45min heating;... | Download Scientific Diagram

Molecules | Free Full-Text | Transesterification of Lactic Acid Oligomers with Ethanol, a Way to Anhydrous Ethyl Lactate: A Kinetic Study
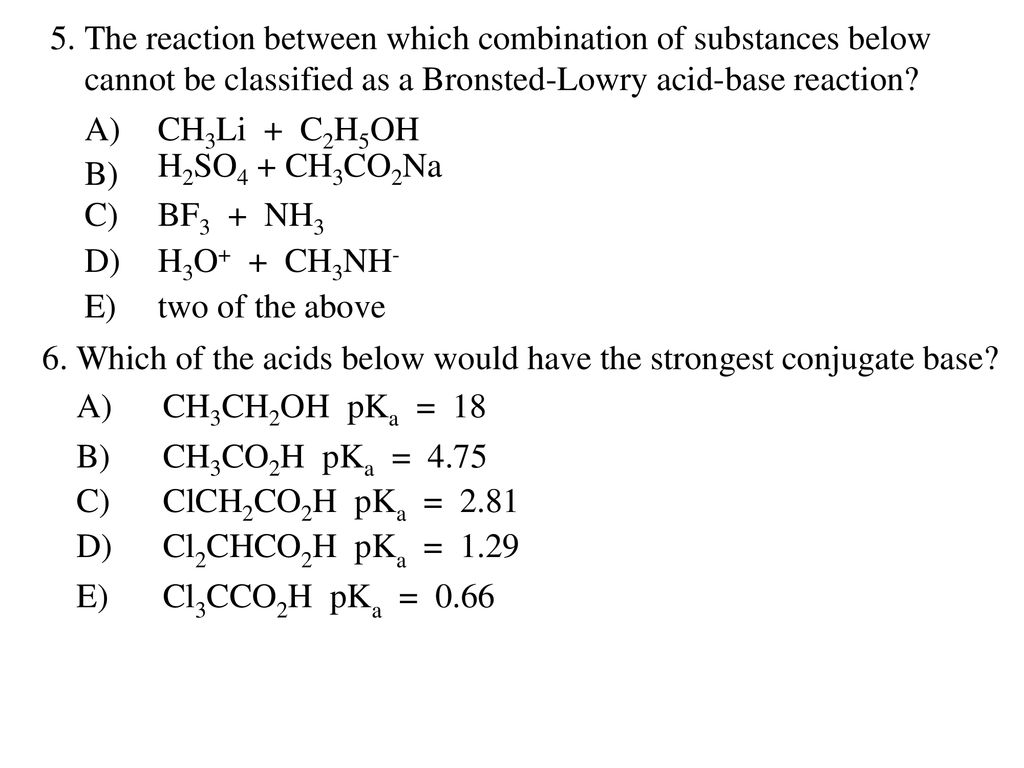
1. Which of the following is not both a Bronsted-Lowry acid and a Bronsted-Lowry base? A) HSO4- (hydrogen sulfate) B) H2PO4- (dihydrogen phosphate) - ppt download
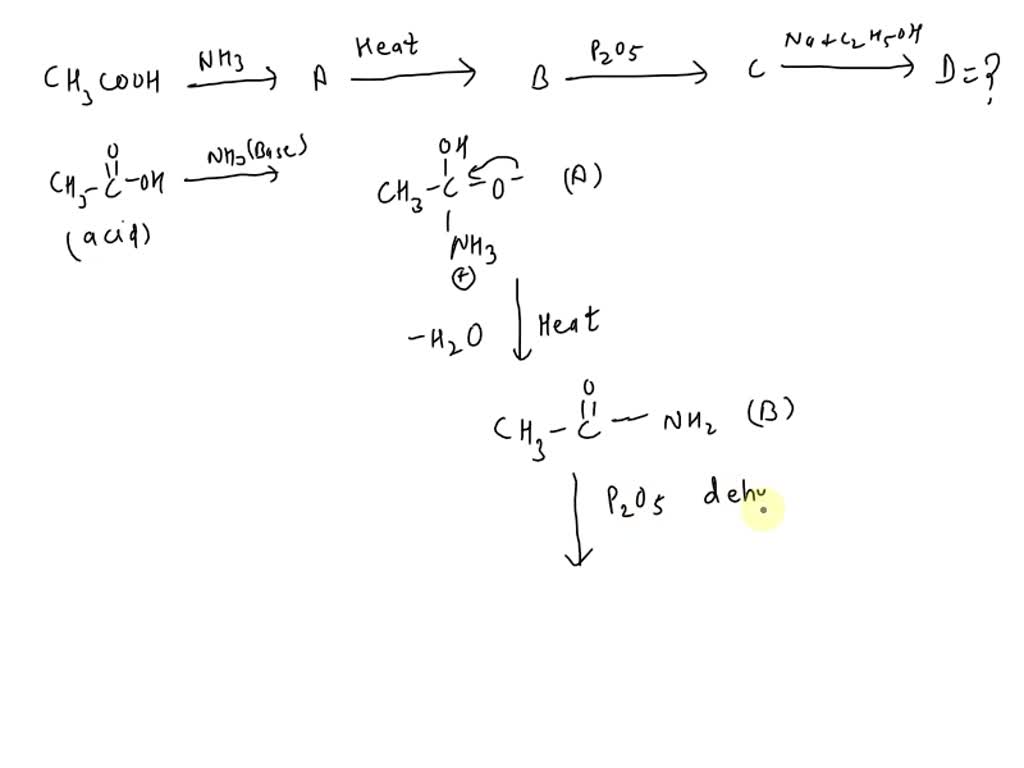
SOLVED: The product (D) in the following sequence of reactions is: CH3COOH NH3 (A) Heat (B) P2O5 (C) Na + C2H5OH (D) A. ester B. amine C. acid D. alcohol

SOLVED: Identify each substance as an Arrhenius acid, an Arrhenius base, or neither. NaOH C2H5OH H3PO4
Which of the following reacts is not shown by formic acid? Reaction with Ca(OH)2 Reaction with I2 / Red P Reaction with NaHCO3 Reaction with C2H5OH